Most smokers interested in quitting do not feel ready for an abrupt cessation, often referred to as “cold turkey.” Most smokers prefer to gradually quit by reducing the number of cigarettes they smoke (Cheong, Yong, & Borland, 2007). This method, however, fails more often than not (Levinson, Shapiro, Schwartz, & Tursky, 1971). It has been hypothesized that nicotine–replacement therapy might aid smoking cessation by permitting smokers to gradually reduce the number of cigarettes they smoke. This week’s ASHES reviews a randomized double-blind placebo-controlled clinical trial that tests the efficacy of nicotine gum to assist cessation (Shiffman, Ferguson, & Strahs, 2009).
Methodology
- Investigators enrolled 3297 participants (57% women) using print and radio advertisements. The investigators randomized participants to nicotine gum or placebo treatment.
- Participants self-selected their nicotine gum dosage (2 mg (n = 1636) vs. 4 mg (n = 1661))
- A user-guide instructed participants to add an additional hour of abstinence and substitute one additional piece of gum daily. Once smokers had quit completely (i.e., for 24 hours), clinicians instructed participants to gradually reduce use of gum consistent with the current FDA-approved
labeling. - The investigators compared the number of smokers who achieved and maintained abstinence—self-reported, verified by two CO readings <= 10 ppm in average—for the placebo condition and for each dose level of nicotine gum.
Results
- As Figure 1 illustrates, smokers using nicotine gum were more likely to achieve initial cessation (i.e., no smoking for 24 hours) compared to the
placebo group. They also were more likely to maintain abstinence 28 days and six months later (p < .05). - Participants using nicotine gum were more likely to experience an adverse event (e.g., nausea, hiccups, heartburn) than participants using placebo gum (48% vs. 37%, p < .001)
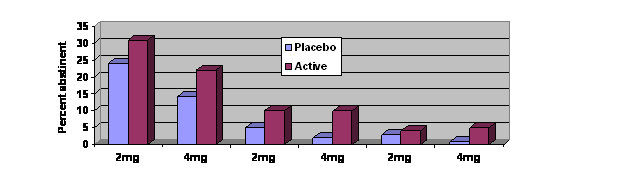
Figure. Initial cessation and continuous smoking abstinence percentages. Initial abstinence 28-day abstinence 6-month abstinence. Click image to enlarge.
Limitations
- Low absolute cessation rates Specific sample (i.e., smokers who are ready to quit on a relatively time-limited program) restricts the ability to generalize these results to other groups.
- The absence of a procedural measure of fidelity reveals that it is not clear, how precisely smokers followed the instructions.
- As Table 1 shows, the study encountered non-completers; it is not evident whether these completers were systematically different from completers; therefore, it is not possible to determine whether the treatment yielded differential impact upon the participants both within and across groups.
|
|
Placebo (N = 1648) |
Nicotine gum (N = |
|
28-day follow-up: number of drop outs |
238 |
262 |
|
6-month follow-up: number of drop outs |
8 |
21 |
Figure. Number of non-completers per condition, summarized across 2mg and 4 mg self-selected groups
Conclusions
Nicotine gum is widely used to help smokers maintain abstinence (Silagy, Lancaster, Stead, Mant, & Fowler, 2004). This study is the first to investigate the effect of nicotine gum on graduated abstinence from smoking. Although the absolute rate of quitting was low, it is comparable to similar “cold turkey” studies (Shiffman et al., 2002). The results suggest that, for smokers quitting gradually, using nicotine gums facilitates tobacco abstinence compared to a placebo. Investigators should conduct more studies to compare its safety and effectiveness against other smoking cessation methods and to include fidelity measure to ensure the participants followed the
instructions.
References
Cheong, Y., Yong, H.-H., & Borland, R. (2007). Does how you quit affect success? A comparison between abrupt and gradual methods using data from the International Tobacco Control Policy Evaluation Study. Nicotine & Tobacco Research, 9(8), 801-810.
Levinson, B. L., Shapiro, D., Schwartz, G. E., & Tursky, B. (1971). Smoking elimination by gradual reduction. Behavior Therapy, 2(4), 477-487.
Shiffman, S., Ferguson, S. G., & Strahs, K. R. (2009). Quitting by Gradual Smoking Reduction Using Nicotine Gum: A Randomized Controlled Trial. American Journal of Preventive Medicine, 36(2), 96-104.e101.
Shiffman, S., Rolf, C. N., Hellebusch, S. J., Gorsline, J., Gorodetzky, C. W., Chiang, Y.-K., et al. (2002). Real-world efficacy of prescription and over-the-counter nicotine replacement therapy. Addiction, 97(5), 505-516.
Silagy, C., Lancaster, T., Stead, L., Mant, D., & Fowler, G. (2004). Nicotine replacement therapy for smoking cessation. Cochrane Database
Systematic Reviews, 3, CD000146.
What do you think? Please use the comment link below to provide feedback on this article.