Two decades ago, multimedia CD-ROMs were the cutting edge of intervention technology; one decade ago, interactive websites were at the forefront; today, “there’s an app for that.” But is there anything particularly effective about the newest intervention modality? Unlike CDs and websites, interventions that use a mobile platform allow messages to reach users rapidly no matter where they are. Continuing the BASIS Special Series on Addiction and Technology, this week’s ASHES reviews a study evaluating a text-message based intervention for smoking cessation (Naughton et al., 2014).
Methods
- Naughton et al. used a randomized controlled trial to compare (1) a conventional smoking cessation intervention delivered within a primary care setting to (2) the combination of the primary care smoking cessation intervention with an SMS-based mobile intervention program, iQuit.
- In the primary care smoking cessation intervention (treatment as usual; TAU), practitioners evaluate patients’ smoking, deliver advice about smoking cessation, agree upon a quit date with their patients, offer cessation aids (e.g., a nicotine patch), and schedule follow-up visits.
- iQuit adds an individually-tailored report and consequent set of text messages to the above intervention. iQuit sends text messages 0-3 times per day, depending on an individualized plan, for 90 days. These messages are tailored to patients’ answers to a set of questions about their smoking; these questions are asked at baseline, 3 weeks, and 7 weeks post-baseline. iQuit also allows users to request texts when they are in potential relapse situations.
- Participants included 602 patients (i.e., 299 in the iQuit condition; 303 in the TAU condition) at 32 general practices in eastern England who smoked cigarettes and were willing to attempt to quit smoking.
- Outcome measures included:
- Past-14 day abstinence, assessed at 4 week follow-up appointment via CO reading;
- Self-reported past-14 day abstinence at 8 weeks;
- Self-reported past-90 day abstinence at 6 months;
- Self-reported past-6 month abstinence at 6 months
Results
- Of the 299 participants who received the iQuit intervention:
- 18.5% used the relapse situation text request feature;
- 18.9% discontinued the receipt of texts at some point during the 90-day trial.
- Figure 1 displays the abstinence rates for participants receiving the conventional primary care intervention (TAU) and those receiving the iQuit individually-tailored messages.
- Participants in the iQuit and TAU conditions did not differ significantly in their abstinence at any given time point.
- Participants in the iQuit condition differed from those in the TAU condition on the two measures of long term abstinence: A greater percentage of participants in the iQuit condition than those in the TAU condition reported 6 month continuous abstinence and qualified for abstinence across all time points.
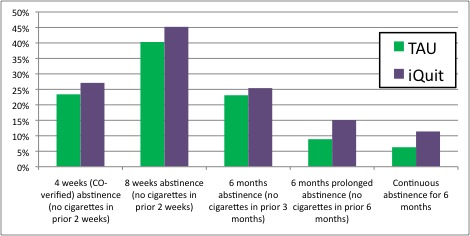
Figure. Abstinence Rates for Patients in the TAU and iQuit Smoking Cessation Conditions. Click image to enlarge.
Note. Continuous abstinence for 6 months is operationalized as qualifying for abstinence on each of the other four abstinence measures. *Difference between conditions, p < .05.
Limitations
- Measures of long term abstinence, on which the conditions differed, relied on self report, which is vulnerable to various biases and recall errors.
- Attrition at each time point ranged from 16-30%. It is possible that those who completed the follow-up measures differed from those who did not in ways that affected study outcomes.
Conclusions
The current study offers evidence that text-message based interventions for smoking cessation might improve smokers’ chances of maintaining abstinence across time. In particular, approximately 20% of the intervention participants in this trial made use of the relapse situation feature of iQuit. The instant availability of help in these instances might be a particularly unique and valuable contribution of mobile platform based interventions such as this one.
— Sarah Nelson
References
Naughton, F., Jamison, J., Boase, S., Sloan, M., Gilbert, H., Prevost, A. T., . . . Sutton, S. (2014). Randomized controlled trial to assess the short-term effectiveness of tailored web- and text-based facilitation of smoking cessation in primary care (iQuit in Practice). Addiction, 109, 1184-1193.
What do you think? Please use the comment link below to provide feedback on this article.