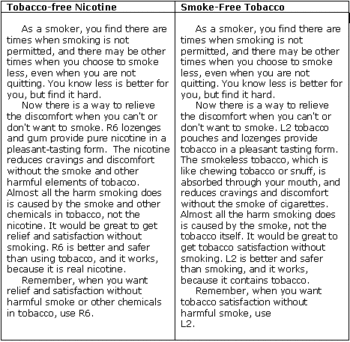
As a result of the many difficulties associated with quitting smoking (e.g., psychological and physiological withdrawal) and even the challenges to reducing smoking (e.g., unintended increase in smoking intensity via deeper puffs), tobacco control experts have recommended the use of pure nicotine products as a “harm-reduction strategy”. Studies show that the use of medicinal nicotine (MN; e.g., nicotine gum, an inhaler) significantly reduces smoking (Bolliger et al., 2000; Wennike, Danielsson, Landfelt, Westin, & Tonnesen, 2003). Alternatively, people occasionally advocate smokeless tobacco (SLT) as another potential aid in smoking reduction; SLT products contain chemical toxins but are arguably less harmful than smoking (Royal College of Physicians of London, 2000). This week’s ASHES reviews an investigation of the comparative appeal of MN and SLT to current smokers.
Shiffman, Gitchell, Rohay, Hellebusch, and Kemper (2007) conducted two studies comparing smokers’ self-reported preference for MN or SLT. In Study 1, the researchers contacted participants via a random-digit-dial telephone interviewing system using numbers from the United States Scientific Telephone Sample; 66% of those contacted completed the survey. The interviewer played current smokers (n=283) a recording of a 1-minute advertisement describing each product (as seen in Table 1), and asked them standard market research questions about which one they preferred. Study 2 followed the same procedure. However, in Study 1, the advertisements introduced prototypical forms of both MN and SLT (e.g., nicotine gum, chewing tobacco, respectively) whereas in Study 2, both products were introduced in a novel manner, as lozenges. Here we only report the findings of Study 1 because the results refer to the more widely known forms of MN and SLT.
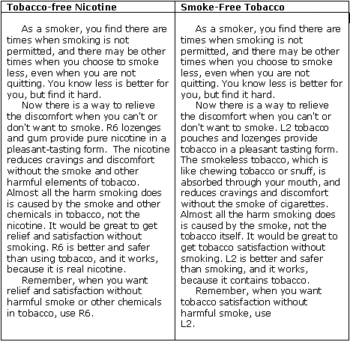
Figure. MN and SLT Readings (adapted from Shiffman et al., 2007). Click image to enlarge.
Analyses indicated participants reported a significant preference for MN: 59% of participants preferred MN whereas only 22% of participants reported preferring SLT (p<0.0001). Previous SLT users (n=69) expressed a greater preference for MN than SLT (44% vs. 39%), as did nonusers (n=214; 64% vs. 17%). However, a chi-square analysis indicated independence between the groups; nonusers’ preference was significantly greater than that of previous SLT users (p=.0003). Both previous MN users (n=37) and nonusers (n=246) preferred MN to SLT (67% vs. 19%, 58% vs. 23%, respectively), but there was no interaction between the groups’ preferences (p=ns).
There are two intertwined limitations of this study. First, participants assessed their preference for a product based only on a 1-minute description; without a more detailed explanation of the product or the opportunity to experiment with it, it is unlikely that participants could form a valid opinion. Second, people’s intended or expected actions often differ from their actual behaviors (Baumeister, Vohs, & Funder, 2007). Therefore, although participants expressed an increased likelihood of using MN, it is possible that given the opportunity, participants would choose SLT or an entirely different option.
The results of this study serve as an initial aid in creating both safe and appealing ways for smokers to obtain nicotine without smoking. Although public health strategies previously encouraged people to quit nicotine consumption altogether, the use of replacement nicotine has been shown to reduce smoking, which reduces the amount of toxins ingested into the body. Further investigations are needed to determine the least harmful and most attractive forms of pure nicotine products before this concept of replacement nicotine can be seriously utilized as a public health strategy.
–Sara Kaplan.
References
Bates, C., Fagerstrom, K., Jarvis, M. J., Kunze, M., McNeill, A., & Ramstrom, L. (2003). European Union policy on smokeless tobacco: A statement in favour of evidence based regulation for public health. Tobacco Control, 12(4), 360-367.
Baumeister, R. F., Vohs, K. D., & Funder, D. C. (2007). Psychology as the science of self-reports and finger movements: Whatever happened to actual behavior? Perspectives on Psychological Science 2(4), 396-403.
Bolliger, C. T., Zellweger, J. P., Danielsson, T., van Biljon, X., Robidou, A., Westin, A., et al. (2000). Smoking reduction with oral nicotine inhalers: Double blind, radnomized clinical trial of efficacy and safety. British Medical Journal, 321, 329-333.
Royal College of Physicians of London. (2000). Nicotine addiction in Britain: A report of the Tobacco Advisory Group of The Royal College of Physicians. London: Royan College of Physicians.
Shiffman, S., Gitchell, J., Rohay, J. M., Hellebusch, S. J., & Kemper, K. E. (2007). Smokers’ preferences for medicinal nicotine vs. smokeless tobacco. American Journal of Health Behavior 31(5), 462-472.
Wennike, P., Danielsson, T., Landfelt, B., Westin, A., & Tonnesen, P. (2003). Smoking reduction promotes smoking cessation: Results from a double blind randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction, 98(10), 1395-1402.
What do you think? Please use the comment link below to provide feedback on this article.