Most everyone in the developed world knows the health effects of tobacco smoke and more than 70% of smokers report that they want to quit (Schroeder & Cox, 2006). Those wishing to quit have a variety of tools to choose from, including, Nicotine Replacement Therapy (NRT) (see ASHES Vol. 3(6)), smoking cessation treatment programs (see ASHES Vol. 3(5), ASHES Vol. 3(4), and ASHES Vol. 2(9)), and drug therapy, which until recently was limited to bupropion SR (ALA, 2006). This week ASHES reviews a clinical trial that studied the safety and efficacy of varenicline, a drug treatment option recently approved by the FDA, to help people with nicotine dependence.
Gonzalez et al. (2006) used a randomized double-blind, parallel-group, placebo-and active-treatment-controlled, phase 3, multi-site clinical trial of varenicline to study its effectiveness in helping people to quit smoking. Participants, recruited through media advertisements, included healthy people 18 to 75 years of age, who smoked at least 10 cigarettes per day, were not abstinent during the previous 3 months, and who had a desire to quit. Researchers randomized 1,025 eligible participants in a 1:1:1 ratio to receive either varenicline 1mg twice daily, bupropion SR 150mg twice daily, or matching placebo for 12 weeks, and then followed participants for 40 weeks. Gonzalez et al. reported similar compliance rates for the groups, with the median treatment time being 84 days. Participant completion rates varied from 60.5% for varenicline, 56% for bupropion SR, and 54% for placebo.
Researchers measured continuous abstinence through exhaled carbon monoxide. As Table 1 shows, at weeks 9-12 and weeks 9-24 the group taking varenicline had a higher continuous abstinence rate than both bupropion SR and placebo. At week 52, the group taking varenicline had a continuous abstinence rate of 21.9% that was no longer statistically different from the 16.1% continuous abstinence rate of the group taking bupropion SR. However, both groups were statistically different from the 8.4% continuous abstinence rate of the placebo group.
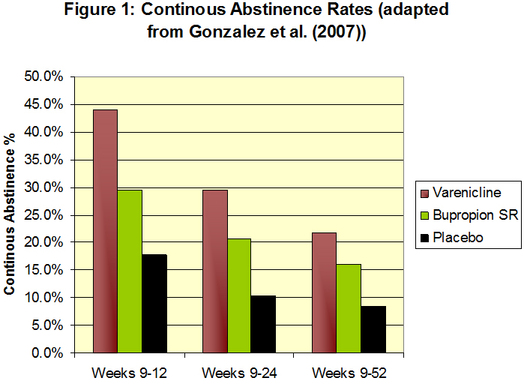
Figure. Continuous abstinence rates adapted from Gonzalez et al. (2007). Click image to enlarge.
Note. P<.001 for all comparisons except varenicline vs. bupropion SR at weeks 9-24 (P=.007),
varenicline vs. bupropion SR at weeks 9-52 (P=.057), and bupropion SR vs. placebo at weeks 9-52 (P=.001).
Limitations to this study include restricting the study to only healthy individuals. The study also excluded those who had previously used bupropion SR for either depression or to help them quit smoking. The researchers did this to study the true difference between the drugs and prevent a negative bias against bupropion SR. Consequently, it is possible that both groups might be more motivated to quit. This extra motivation might increase the continuous abstinence rate.
Despite these limitations, this study suggests that, for some, varenicline is an effective treatment for smoking cessation. Results showed that 22% of participants who used varenicline were able to remain abstinent for the full 52 weeks of the study, a rate two-and-a-half times that of the placebo. Importantly, the results indicate that people in the varenicline group quit smoking more quickly. Improving health more quickly is an important effect even if over the long-term rates of improvement equal out. That said, there is no cure-all, no magic pill for everyone, only options that people should use with trial and error until they are able to maintain, in the case of tobacco, abstinence. When discussing smoking cessation options, doctors, psychiatrists, and patients should remain open to other forms of treatment or consider taking varenicline in concert with nicotine replacement, counseling, or online help. Although quitting smoking is a difficult task, it is possible, and many people succeed everyday. Perhaps most importantly, they succeed using a variety of methods.
–John Kleschinsky.
References
ALA. (2006). Nicotine Replacement Therapy (NRT) and Other Medications Which Aid Smoking Cessation, Quit Smoking.
Gonzales, D., Rennard, S. I., Nides, M., Oncken, C., Azoulay, S., Billing, C. B., et al. (2006). Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. Jama, 296(1), 47-55.
Schroeder, S. A., & Cox, H. C. (2006). Trials that Matter: Varenicline: A Designer Drug to Help Smokers Quit. Annals of Internal Medicine, 145(10), 784-785.
What do you think? Please use the comment link below to provide feedback on this article.




Aleena February 23, 2014
When doing this experiment did any of the participants suffer from any side effects?