As awareness about and use of e-cigarettes has grown across the globe (ASHES Vol. 9(6)), these devices have come under more scrutiny from researchers and public policy experts. E-cigarettes are gaining popularity because like nicotine patches, they provide a low dose of nicotine, contain fewer toxins than cigarettes, and simulate behavioral and sensory dimensions of smoking. However, because e-cigarettes are a relatively new technology, there is a paucity of research to inform discussions about safety and efficacy. This week’s ASHES reviews a recent randomized control trial examining the efficacy of e-cigarettes compared to placebo and nicotine patches for smoking cessation (Bullen et al., 2013).
Methods
- Researchers recruited a sample of 657 New Zealand adult smokers who wanted to quit smoking cigarettes.
- Participants were randomized into 3 groups:
- 289 received e-cigarettes;
- 295 received nicotine replacement patches; and
- 73 received placebo e-cigarettes (i.e., a vaporized solution with no nicotine)
- Participants were randomized into 3 groups:
- Participants completed telephone interviews at baseline, and follow-up surveys at 1, 3, and 6 months.
- Outcomes of interest included cigarette smoking abstinence, number of cigarettes per day, and adverse events related to e-cigarette or nicotine patch use (e.g., persistent or significant disability, admission to hospital).
- Researchers used intent to treat analysis and compared groups using Chi square and multivariate regressions.
Results
- As Figure 1 shows, e-cigarette users initially had better success maintaining continuous abstinence at one month than patch users. This difference is no longer significant at 3 months and 6 months. There was not enough statistical power to detect differences in continuous abstinence between e-cigarettes and placebos.
- Among those who relapsed (i.e., began smoking cigarettes again), the median time to relapse was 35 days among the e-cigarette group, 2.5 times longer than the nicotine patch group, and nearly 3 times longer than the placebo group.
- At all time points, e-cigarette users who did relapse reported smoking significantly fewer cigarettes per day than patch users who relapsed.
- There was no significant difference in the rate of serious adverse events between groups (i.e., 19.7% for e-cigarette group; 11.8% for nicotine patch group; and 13.9% for placebo e-cigarette group).
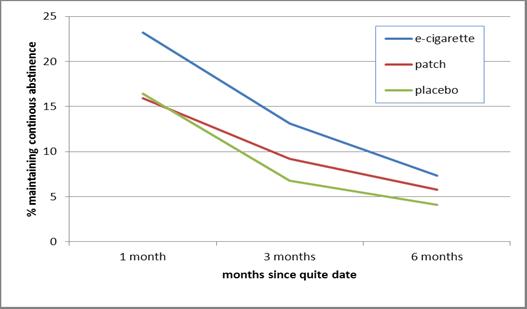
Figure. Percent maintaining continuous cigarette smoking abstinence by time and treatment group. Click image to enlarge.
Limitations
- Rates of smoking abstinence at 6-month follow-up were much lower than anticipated, leading to insufficient power to detect differences between groups, particularly between the placebo group and other groups.
- Most of the outcomes assessed (e.g., cigarettes per day, continuous abstinence) relied on self-report and are therefore subject to memory errors and other limitations.
Conclusion
The efficacy of e-cigarettes to help individuals quit smoking remains unresolved. This study suggests that e-cigarettes are as effective as the patch in terms of abstinence at 6-months, and appear to be more effective initially. Researchers also found some promising results including a longer time to relapse and larger reductions in the number of cigarettes smoked per day among e-cigarette users compared to patch users. In addition, e-cigarette users reported adverse events at similar rates compared to the other two groups. Future research should attempt to replicate the study with larger samples and varying nicotine doses.
–John Kleschinsky
References
Bullen, C., Howe, C., Laugesen, M., McRobbie, H., Parag, V., Williman, J., & Walker, N. (2013). Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet, 382(9905), 1629-1637. doi: 10.1016/S0140-6736(13)61842-5
What do you think? Please use the comment link below to provide feedback on this article.