Ketamine (street name “Special K”) is an anesthetic drug that some people use recreationally at sub- anesthetic doses to induce a dissociative state. Notably, some research suggests that intravenous ketamine provides rapid relief from depressive symptoms among people with major depressive disorder (MDD). Ketamine might therefore be a useful option for people who have not responded to traditional anti-depressives (Berman et al. 2000). This week’s STASH reviews meta-analysis describing studies of ketamine for the treatment of MDD and bipolar disorder (BD) (aan het Rot, Zarate Jr., Charney, & Mathew, 2012).
Methods
- To identify studies, the researchers searched for relevant terms in scientific databases and examined reference lists.
- Inclusion criteria included:
- Patients met clinical criteria for a mood disorder.
- Patients received ketamine to study its antidepressant effects.
- Patients were clinically assessed for at least 230 minutes after they received ketamine.
- Inclusion criteria included:
- The search yielded 10 case studies (total N = 14), 10 open-label investigations (total N = 83), and 5 controlled trials (total N = 66). Here we focus on the 2 open-label investigations and 5 controlled trials that measured response up to 72 hours post infusion.
- Patients in different studies had different levels of resistance to depression treatment; however, no studies included patients using ketamine as an initial treatment for depression.
- The researchers defined a “response” to ketamine as a 50% or greater improvement on at least one of these self-report measures of depression:
- Antidepressant Treatment History Form (ATHF)1
- Beck depression Inventory (BDI) 2
- Hamilton Depressive Rating Scale (HDRS)3
- Montgomery-Asberg Depression Rating Scale (MADRS)4
- Using this definition, the researchers identified the percentage of participants classified as responders in each study at each time point (i.e. 3 to 4, 24, and 72 hours after infusion).
Results
- Most studies used one dose of .5mg/kg of ketamine administered intravenously (IV) over 40-60 min with a placebo of saline in a randomized order.
- Figure 1 shows IV ketamine response among participants in open-label and controlled studies. Ketamine had rapid, but transient, antidepressant effects and effects varied considerably from study to study.
- At 3-4 hours, the percent of patients responding ranged from 0-100%. At 72 hours, the percent of patients responding ranged from 14-70%.
- Adverse effects during administration included dissociation, dizziness, and blood pressure changes.
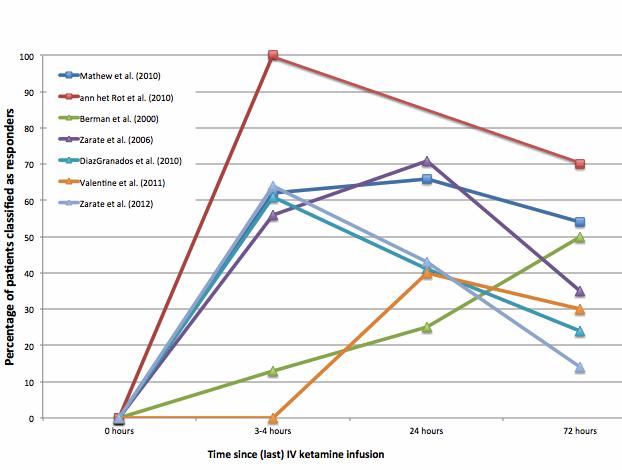
Figure. Percent of patients who responded to IV ketamine over time in open-label (square markers) and controlled (triangle markers) trials organized by disorder treated, type of trial, and number of doses (Adapted from aan het Rot et al., 2012). Click image to enlarge.
Limitations
- The total number of patients is small.
- Inter-study variations in ketamine amount and administration methods, as well as outcome data, make it difficult to compare outcomes of the different studies.
- Outcome assessments were very short in duration; the longest follow-up period was only 72 hours.
- This study did not clearly outline the selection criteria of patients included in the various studies.
Conclusions
Despite its misuse potential in recreational settings, there seem to be benefits in using ketamine in controlled settings for people who do not respond to anti-depressants. These data suggest fast-acting but somewhat transient effects of IV ketamine; future studies are needed to determine whether ketamine can provide long-lasting effects, potentially using other delivery modes (e.g., oral, intranasal, intramuscular). Patients varied a great deal in their response to treatment, suggesting the need for additional research on predictors of response. Lastly, researchers are using animal models and brainwave recordings to gather important information on the neurobiological mechanisms underlying ketamine’s effects (e.g., Duman & Aghajanian, 2012).
–Emily Shoov
What do you think? Please use the comment link below to provide feedback on this article.
References
aan het Rot, M., Zarate Jr., C. A., Charney, D.S., Mathew, S.J. (2012). Ketamine for depression: Where do we go from here? Biological Psychiatry 72(7), 537–547.
Berman, R. M., Cappiello, A., Anand, A., et al. (2000). Antidepressant effects of ketamine in depressed patients. Biological Psychiatry, 47, 351-354.
DiazGranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. (2010): A randomized add-on trial of an N-methyl-D aspartate antagonist in treatment-resistant bipolar depression. Archives of General Psychiatry, 67, 793– 802.
Duman, R. S. & Aghajanian, G. K. (2012). Synaptic dysfunction in depression: Potential therapeutic targets. Science, 338 (6103), 68-72.
Mathew, S. J., Murrough J. W., aan het Rot, M., Collins, K A., Reich, D. L., & Charney, D. S. (2010). Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: A pilot randomized, placebo-controlled continuation trial. International Journal of Neuropsychopharmacology, 13, 71-82.
Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, et al. (2011): The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Research, 191, 122–127.
Zarate CA, Brutsche NE, Ibrahim L, Franco-Chaves J, DiazGranados N, Cravchik A, et al. (2012): Replication of ketamine’s antidepressant efficacy in bipolar depression: A randomized controlled add-on trial. Biological Psychiatry, 71, 939 –946
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. (2006): A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry, 63, 856–864.
________________
[1] An instrument designed to assess adequacy of medication trials for major depression by rating them on a scale of 1-5, now in the form of a computer-based algorithm (Oquendo, 2003)
[2] A 21-item self-report of depressive ideation (Beck, 1961)
[3] A multi-item questionnaire used to provide an indication of depression (Hamilton, 1960).
[4] A ten-item diagnostic questionnaire used to measure severity of depressive episodes in patients with mood disorders. (Montgomery and Asberg, 1979)




