Relapse represents one of the major hurdles in the long-term treatment of addictive disorders. In addition to behavioral therapies, pharmacotherapy is proving to be a valuable component in relapse prevention. Whereas the study of rats has provided valuable insight into possible causes of addiction in humans (see WAGERs 8(35) and 8(36)), the study of pharmacological treatment of alcohol and drug relapse behaviors in rats has also yielded possible parallels with human relapse behaviors. This week The WAGER presents an article by Le and Shaham (2002) that reviewed research on neurobiological and pharmacological aspects of alcohol relapse in rats.
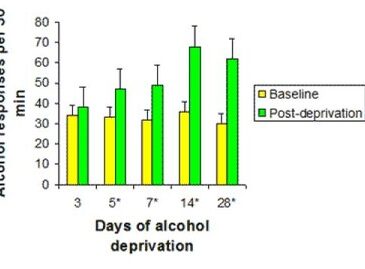
Le and Shaham (2002) noted that many studies of alcohol relapse in rats utilize a specialized alcohol-deprivation control model. The alcohol-deprivation model examines the effect of abstinence on alcohol consumption at relapse. In the alcohol-deprivation model, scientists allow rats unrestricted access to alcohol for various periods of time and then remove the alcohol completely. They allow the rats to experience withdrawal for different time periods before reintroducing alcohol. Le and Shaham (2002) reported that an alcohol deprivation effect (ADE), a temporary (approximately 48 hour) increase of alcohol consumption over baseline levels after a period of abstinence, was repeatedly observed in such studies (see Figure 1 for an example). The studies reviewed by the authors utilized this model to compare rats’ behavior (amount of alcohol consumed post-deprivation) between the control conditions and when treated with various pharmacological substances intended to block the effects of alcohol.
Figure 1. Example of alcohol-deprivation effect (ADE) in rats (Heyser, Schulteis, & Koob, 1997, as cited in Le & Shaham, 2002)
* Indicates significant difference between baseline and post-deprivation, p < 0.05.
The control scenario measures the unimpeded (i.e., untreated) behavioral aspects of alcohol relapse; Le and Shaham (2002) linked increased alcohol consumption behavior to neurobiological origins. In their review of the literature on pharmacological aspects of alcohol relapse, the authors reported that several studies indicated that opioid receptors in the brain could be involved in the mechanisms underlying re-exposure-induced alcohol relapse: in such situations, opioid receptor antagonists (pharmacological substances that block the physical effects of drugs) effectively reduce the alcohol-deprivation effect (ADE) in rats. The authors also found that multiple studies linked the mesolimbic dopamine system with re-exposure-induced relapse, and that re-exposure to alcohol can trigger ADE through increased release of dopamine in the nucleus accumbens—an area of the brain known to be closely associated with addictive behaviors (next week’s WAGER will present more information on this topic).
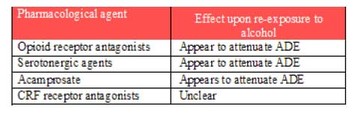
Le and Shaham’s review also reported on studies that tested the effects of other pharmacological agents in combating alcohol relapse in rats. Table 1 provides a summary of their findings.
Table 1. Summary of pharmacological treatments for the prevention of alcohol relapse in rats based on available data (adapted from Le & Shaham, 2002)
The alcohol relapse patterns exhibited by rats and the neurobiological underpinnings of such behaviors could have wide implications for general addiction relapse among humans. Applied to pathological gambling (PG), the animal model appears to parallel human behavior: prolonged abstinence from gambling can result in intense and destructive gambling sessions when relapse occurs. However, while pharmacotherapy has yielded positive results in preventing addiction relapse in rats, there is a major difference between the animal research environment and the human environment. While rats have no choice as to when they will be given access to alcohol, pathological gamblers are able to access casinos at all times. The impracticality of forced abstinence adds a higher level of complexity to the study of addiction relapse in humans, and presents a challenge to the pharmacological treatment of PG.
The growing body of research on the pharmacological treatment of alcohol and drug relapse in rats could lead to a better understanding of addiction in general, and hence inform our existing knowledge of pathological gambling (PG). Although human studies on the pharmacological treatment of PG are now becoming available (e.g., Kim & Grant, 2001; Kim, Grant, & Adson, 2001) further research is necessary before distinct pharmacological treatment strategies for preventing PG relapse can become a reality. Continued concentration on the reward pathways that are triggered by gambling activity could eventually lead to the identification of specific chemicals and receptor sites involved in producing gambling urges, and could ultimately yield a pharmacological arsenal capable of preventing relapse among pathological gamblers.
Comments on this article can be addressed to Tony Donato at wager@hms.harvard.edu.
References
Heyser, C. J., Schulteis, G., & Koob, G. F. (1997). Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcoholism: Clinical & Experimental Research, 21, 784-791.
Kim, S. W., & Grant, J. E. (2001). An open naltrexone treatment study of pathological gambling disorder. International Clinical Psychopharmacology, 16, 285289.
Kim, S. W., Grant, J. E., & Adson, D. E. (2001). Double-blind naltrexone and placebo comparison study In the treatment of pathological gambling. Biological Psychiatry.
Le, A. D., & Shaham, Y. (2002). Neurobiology of relapse to alcohol in rats. Pharmacology & Therapeutics, 94, 137-156.
The WAGER is a public education project of the Division on Addictions at Harvard
Medical School. It is funded, in part, by the National Center for Responsible
Gaming, the Massachusetts Department of Public Health, the Substance Abuse and
Mental Health Services Administration, and the Center for Substance Abuse
Treatment.