To prevent opioid relapse, doctors can turn to medication assisted treatment (MAT), which often includes combining counseling with medications such as methadone, Buprenorphine, or extended-release naltrexone. Previous research with criminal justice offenders has shown extended release naltrexone to reduce relapse by about 30%. However, little is known about how extended release naltrexone, a newer form of treatment, compared to more traditional forms of treatment, such as Buprenorphine for opioid relapse prevention within the general population. This week’s STASH reviews a study by Dr. Joshua Lee and his colleagues that assessed the effectiveness of both extended-release naltrexone and Buprenorphine-naloxone for opioid relapse.
What was the research question?
Which is more effective for opioid relapse prevention: extended-release naltrexone or Buprenorphine-naloxone?
What did the researchers do?
During a 24 week study period, the researchers conducted an open-label randomized controlled trial at 8 community treatment programs. The study included 570 adults who had used opioids in the past month and met DSM-V diagnostic criteria for an opioid use disorder. To study effectiveness, the researchers assessed if participants successfully completed the induction phase of treatment (i.e., the beginning of medication-assisted treatment after withdrawal and before the maintenance phase) and if participants experienced a relapse during the 24 week study period.
What did they find?
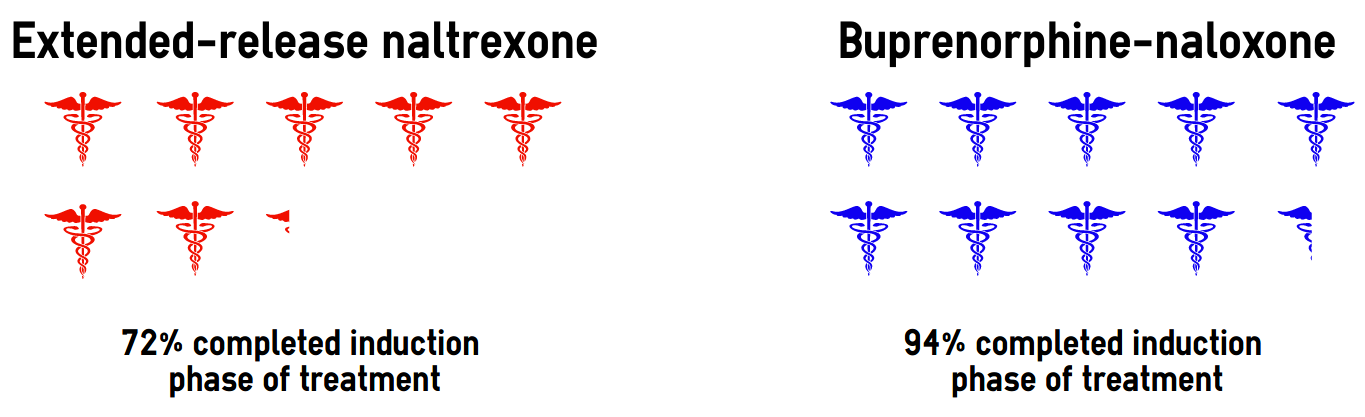
As displayed in Figure 1, Lee and his colleagues found that participants in the extended-release naltrexone group had a harder time completing the induction phase of treatment compared to Buprenorphine-naloxone. 72% of participants in the extended-release naltrexone group completed induction, compared to 94% in the Buprenorphine-naloxone group. In addition, participants in the extended-release naltrexone treatment group were also approximately 44% more likely to experience a relapse compared to those being treated with Buprenorphine-naloxone generally. Lee and his colleagues reported that most of that difference was due to relapse during the induction phase of treatment.
Figure. Percentage of treatment seekers for an opioid use disorder who successfully completed the induction phase of treatment by medication type, adapted from Lee et al., 2017. Click image to enlarge.
Why do these findings matter?
These findings indicate a need for treatment providers in community settings to better support induction among patients using extended-release naltrexone to treat an opioid use disorder. This might explain why previous studies have shown positive results with extended-release naltrexone in controlled settings. Those seeking help for an opioid use disorder outside of controlled settings should consider medication-assisted treatment with Buprenorphine-naloxone.
Every study has limitations. What are the limitations in this study?
Both treatment providers and treatment seekers were aware of the medications they were taking, which might introduce bias into the results. The authors note that the varied induction protocols at the treatment centers might have had a substantial effect on participants successful induction with extended-release naltrexone. This methodological challenge limits our ability to interpret the findings. Finally, the acute detoxification setting limits our ability to generalize this study’s findings to other types of treatment centers.
For more information:
If you are concerned about yours or a loved one’s substance use, visit our resource page for brief screens to assess substance use and self-help tools.
— John H. Kleschinsky
What do you think? Please use the comment link below to provide feedback on this article.




David Wilson November 29, 2017
This is a troubling and misleading comparison for those who do understand that buprernorphine is an opiate from which patients will eventually need to be detoxed with all of the liabilities that exist from an opiate detox, even recognizing that this detox may be a bit easier than from illicit opiate use. Still a detox will be needed if patients wish to discontinue this medication. Naltrexone is non-addictive and requires no further detox to discontinue. Add to this the caveat, “…the varied induction protocols at the treatment centers might have had a substantial effect on participants successful induction with extended-release naltrexone” and the comparison becomes even more problematic.
A more appropriate comparison might be naltrexone vs drug free treatment. Then the value of naltrexone could be better assessed.
I’m not more in favor of one or the other. I’ve worked regularly with both in my practice and help patients decide which would be a better choice on an individual basis. My clinical impression has been that the profile and differences between those who choose one or the other is considerable. Focusing on the fact that both are nominally “medications” misses and obscures the substantial differences between them.
David Wilson November 29, 2017
Does a naltrexone patient relapse indicate:
1. Opiates detected in system but still continuing with naltrexone? or,
2. Opiates detected in system but discontinued in advance with naltrexone?
My experience has been that the two are often treated as of they are the same, but naltrexone in the system will prevent the high, so the two “relapses” are not the same.
An explanation would be helpful here.
David Wilson December 3, 2017
I found the results and interpretation of this study troubling, so I followed the links offered to the study itself. The findings and interpretation offered suggest that the description offered here by basisonline should be rewritten. In particular, both directly copied from the online report by the authors:
1. Among participants successfully inducted (per-protocol population, n=474), 24 week relapse events were similar across study groups (p=0·44). Opioid-negative urine samples (p<0·0001) and opioid-abstinent days (p<0·0001) favoured BUP-NX compared with XR-NTX among the intention-to-treat population, but were similar across study groups among the per-protocol population.
2. INTERPRETATION:
In this population it is more difficult to initiate patients to XR-NTX than BUP-NX, and this negatively affected overall relapse. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
Hence, both are effective.
Induction and selection are the keys to successful use of XR-NTX and BUP-NX. In my clinical experience, use of either is complicated by the provision of only minimal and superficial counseling over the short and long term.
John H Kleschinsky December 5, 2017
Hi David,
Thank you for reading The BASIS and for providing some thoughtful feedback on my review. I’m going to do my best to reply to each comment in order.
I agree with the authors that although these are distinct forms of treatment, extended-release naltrexone being an antagonist and buprenorphine being a partial agonist the comparative data will be helpful to the field. It is worth noting that extended release naltrexone has already been compared to placebo and to treatment as usual. You are correct in pointing out varying induction protocols as a study limitation. I hope that limitation and the overall findings raises awareness about the need for the use of evidence-based strategies for induction.
They assessed relapse at any point after day 20 post-randomization. They defined relapse as the use of non-study opioids. Participants were identified as having relapsed if any of the following occurred: a positive screen for non-study opioids on a urine test or failure to provide urine for screening; 7 consecutive days of self-reported non-study opioid use; or 4 consecutive weeks with any non-study opioid use. To your specific question about naltrexone patient relapse, I think both types of relapse are captured based on my reading of how they assessed for relapse.
I’m glad to see you went back to the source materials for this study review. We make sure to include a link to at least the abstract, and where available the full publicly available article for those who want to dig deeper into the studies that we review at The BASIS. For the sake of brevity, we try to focus on one or two findings from each study. For this review I chose to focus on induction challenges and relapse with a focus on the intention-to-treat analysis (including in the analysis those who relapsed during induction) and not the per-protocol analysis (removing from analysis those that relapsed during induction) that you highlighted. Reason being, I was interested to report on induction in a community setting with these two treatment options. What I’ve seen and heard about extended-release naltrexone in the past focused on treatment in the criminal justice system, which is a more controlled setting. The title and study are framed, to borrow a phrase from a colleague, as a horse-race. That’s probably not the most constructive way to think about it, which gets back to one of your earlier comments. Having reviewed this article and read your comments, I believe both have a role to play in treating an opioid use disorder. However, we cannot ignore the fact that the extended-release naltrexone group experienced four and a half times as many incomplete inductions compared to the buprenorphine-naloxone group. I believe it is fair to include those in the analysis assessing effectiveness of each drug. Look forward to reading more of our perspective David.
Thanks – John
David Wilson December 8, 2017
Hi John,
Thanks for responding to my comments.
First, “horse race” treatment comparisons are deeply troubling because they conflate non-comparable methods. Statistics generated confuse potential consumers and stake holders who focus on the results alone. A horse, a dog, and a man can race, but do the results tell us anything?
Second, “treatment as usual” vs extended-release naltrexone is interesting, but it still lacks the head to head “horse race” of equals that identical treatments with and without extended-release naltrexone might yield.
Third, opiate use with extended-release naltrexone in the system may be seen as an opiate relapse, but the effects of the opiate are blocked; the high is not experienced. One of the greatest challenges in extended-release naltrexone treatments comes from drug treatment staff who ignore this in favor of “a relapse is a relapse.” This notion is reinforced in your response. Buprenorphine-naloxone patients in my practice report consistently that they still can get high from illicit opiate use, a somewhat diminished high but still a high. Extended-release naltrexone patients cannot, and this is far from an inconsequential fact. Responding in treatment to each type of relapse is substantially different.
Fourth, buprenorphine-naloxone patients will eventually have to be detoxed, and this is an opiate detox with all of the liabilities of any opiate detox. Focusing on the short-term results obscures this. Extended-release naltrexone patients will not have to be detoxed. Either can relapse after the medication is discontinued, but not having to come off opiates tilts in favor of the extended-release naltrexone patients. Both medications help outpatient opiate treatments in ways that regular drug-free treatment cannot. Consumers, stake holders and treatment staff need to understand the significant differences that exist in all phases of treatment with them to make the decisions that are needed to transition to lives without illicit opiate use.