As discussed in preceding WAGERs, gambling tasks activate certain areas of the brain and initiate reward pathway processes. This results in short-term changes in the brain that produce immediate feelings of pleasure. However, recent evidence suggests that repeated engagement in rewarding behaviors might also result in long-term brain changes. These long-term brain changes might contribute to lasting behavioral changes, such as addictive behavior. Nestler, Barrot and Self (2002) recently reviewed the evidence pertaining to the role of the .FosB transcription factor in the development of chemical and behavioral addictions. Transcription factors are proteins that regulate the expression of genes, which in turn initiate particular cascades of neural events.
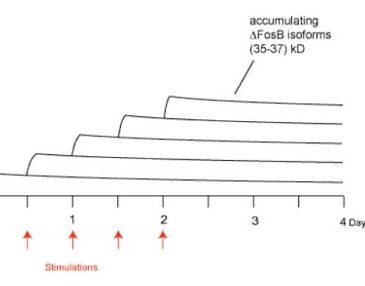
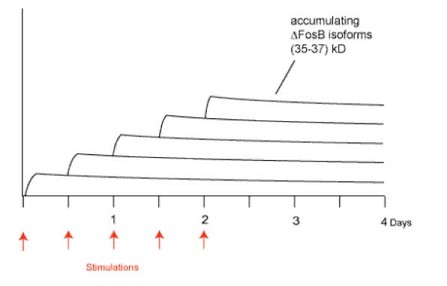
Researchers have observed that .FosB accumulates in certain areas of the human brain after drug use (Nestler et al., 2002) and have suggested other compulsive behaviors (e.g., pathological gambling) might produce the same effect. Some research supports this hypothesis. In an animal model of compulsive behavior, rats that show compulsive running behavior demonstrate a similar accumulation of .FosB (Werme et al., 2002). .FosB is interesting to researchers and treatment providers because while neurotransmitters and other elements involved in the reward pathway disappear rapidly after stimulation (e.g., drug activity) ceases, research has shown an altered form of .FosB persists for several weeks in rats’ brains (Chen, Kelz, Hope, Nakabeppu, & Nestler, 1997). Consequently, when an individual engages in the behavior again, newly produced .FosB is added to the existing .FosB. The result of repeated stimulation (i.e. chronic abuse) is a gradual increase in the total amount of .FosB in the brain that persists for long periods of time (Figure 1). For example, repeated gambling might actually cause a switch in the brain to turn on behaviors we associate with pathological gambling. Since the switch stays on, the urge to gamble remains long after a pathological gambler might have quit gambling.
Figure 1. Gradual accumulation of DFosB isoforms after repeated stimulation (adapted from Nestler et al., 2002).
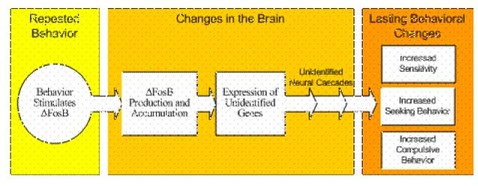
But, what does .FosB do and what is the impact of accumulated .FosB? Scientists know .FosB is stimulated by rewarding behaviors and they are presently attempting to determine its function in the brain. While scientists do not know what specific genes are regulated by the .FosB transcription factor, animal studies provide a roadmap for behavior stimulated .FosB activity (Figure 2). After repeated behaviors, such as gambling, stimulate the production and accumulation of .FosB, .FosB then turns on particular genes. These genes initiate neural cascades that result in lasting behavioral changes that contribute to addiction. Some lasting behavioral changes resulting from .FosB accumulation include: an increase sensitivity to drugs (Kelz et al., 1999; Kelz & Nestler, 2000) and enhanced drug-seeking behaviors (Whisler, Chen, Nestler, & Self, 1999). The consequences of this are obvious.
Figure 2. A potential DFosB mediated pathway by which chronic stimulation might result in sustained behavioral changes.
Determining whether repeated behaviors like gambling are capable of producing a lasting change in brain chemistry is crucial to furthering scientific understanding of addiction. Similar processes across chemical and behavioral addictions suggest a common root. The research reviewed above suggests that behaviors, as well as chemicals, hold the potential to initiate addictive behavior and that .FosB might facilitate this transformation. Strikingly, the behavioral changes instigated by .FosB appear to be longer lived than the transcription factor itself: .FosB is no longer detectable several months after the stimulation ceases yet the sensitivity and seeking behaviors persist for years. This has lead scientists to speculate that there is a hidden step, or “switch,” in the .FosB-initiated cascade that results in a permanent change in gene regulation or neural structure.
Future efforts should be aimed at identifying the mechanism by which .FosB initiates permanent brain changes. Filling in the middle pieces of the puzzle – the genes .FosB regulates and their effects – will help clarify the role of .FosB in forming addiction.
Comments on this article can be addressed to Rachel Kidman.
References
Chen, J., Kelz, M., Hope, B., Nakabeppu, Y., & Nestler, E. (1997). Chronic Fos-related antigens: stable variants of .FosB induced in brain by chronic treatments. Journal of Neuroscience, 17(13), 4933-4941.
Kelz, M., Chen, J., Carlezon, W., Whisler, K., Gilden, L., Beckmann, A., et al. (1999). Expression of the transcription factor .FosB in the brain controls sensitivity to cocaine. Nature (London), 401(6750), 272-276.
Kelz, M., & Nestler, E. (2000). .FosB: a molecular switch underlying long-term neural plasticity. Current Opinions in Neurology, 13(6), 715-720.
Nestler, E., Barrot, M., & Self, D. (2002). DFosB: A sustained molecular switch for addiction. Neural Signaling, 98(20), 11042-11046.
Werme, M. C., Olson L, Gilden L, Thoren P, Nestler EJ, Brene S. (2002). Delta FosB regulates wheel running. Journal of Neuroscience, 22(18), 8133-8138.
Whisler, K., Chen, M., Nestler, E., & Self, D. (1999). Soc. Neuroci. Abstr., 25(811).




Evelyn Wangari June 5, 2017
Educative…