Neuroanatomical Models of Addiction
In preceding issues, our special WAGER series on biology, addiction, and gambling illustrated the importance of the brain’s reward system to the development and maintenance of behavioral addictions. This week’s WAGER focuses on decision making research by Antione Bechara (Bechara, 2003) in the context of its neuroanatomical correlates. Bechara investigates one of the fundamental characteristics of behavioral addictions, an apparent “myopia” for future consequences (i.e., disregard for the health, psychological, and social costs of one’s actions). In our review, we will explain how substance dependence and pathological gambling might in part stem from neuroanatomical deficits in decision making processes.
Since the 1800’s, researchers have found that brain damage (lesions) to the orbital prefrontal cortex (PFC) has been associated with severe impairments in personal and social decision making (Harlow, 1868) such as impulsiveness, irritability, disinhibition of instinctual actions, disregard for social and moral principles, and reckless, risk-taking behavior (Fuster, 2001). Recent research has discovered abnormalities in the PFC of substance dependent individuals (Childress et al., 1999) and pathological gamblers (Potenza et al., 2003). The developmental causes and mechanisms for these abnormalities are not yet clearly understood. However, the behavior that appears to be associated with abnormal PFC functioning within both substance dependent individuals and pathological gamblers is similar to that of PFC-lesioned patients, as they often deny or do no not realize that they have a problem and ignore the future negative consequences of their actions.
In order to measure to the behavioral effects of neuroanatomical decision making impairments, Bechara developed “the gambling task” (GT), a computer game which engages subjects to make advantageous choices by choosing between four decks of cards (see WAGER, Vol.7, Issue.43). The goal of the GT is to maximize profits on a loan of play money. Players are required to choose between immediate monetary reward decks, which have a high-risk of incurring negative consequences, and delayed monetary reward decks, which slowly accrue positive earnings. The GT mirrors real-world contingencies of decision making, as a successful player must learn to avoid large, immediate rewards associated with severe negative consequences, in favor of lower-risk, delayed rewards.
Healthy controls generally perform well on the GT, making decisions which maximize their long-term profits. PFC-lesioned patients, substance dependent individuals, and pathological gamblers play the GT with varying success. PFC-lesioned patients uniformly perform the worst, while the performance of individuals with drug or gambling addictions varies: some perform as well or nearly as well as healthy controls, while others mirror the poor performance of PFC-lesioned patients. These mixed results might indicate differential localization or severity of impairment. The complexity of addiction evidenced in substance dependent and pathological gambling populations appears to corroborate such an explanation.
Bechara suggests that poor performance on the GT reflects a somatic state impairment (see WAGER, Vol.4, Issue.34), signaling a damaged or abnormally functioning ventromedial area of the PFC. However, Bechara’s supposition that the ventromedial area acts as the primary region responsible for decision making is based on a limited number of patients, all of whom sustained inexact brain damage to the PFC. Stronger evidence in human neuropsychology and lesion studies with non-human primates has implicated the dorsolateral PFC in playing a greater role in decision making (Fuster, 1997; Goldman-Rakic, 2000). The dorsolateral PFC is responsible for the temporal organization of behavior (Fuster, 2001). Decision making behavior involving delayed consequences is greatly affected by abnormalities in this area. Further, ascribing activities to one specific brain region might underestimate the complexity of neural functioning. Because the PFC is connected to the basal ganglia, brainstem, limbic system, and thalamus through a myriad of neurotransmitter pathways, none of its cognitive functions can be understood if taken out of a broad, connectionist context (Fuster, 2001). Disruptions within the PFC (e.g., physical damage, neuromodulating drugs, etc.) create detrimental effects that carry over to many interconnected regions, and vice-versa (see Figure 1).
Figure 1. Neurotransmitter pathways through the limbic system (enlarged detail)
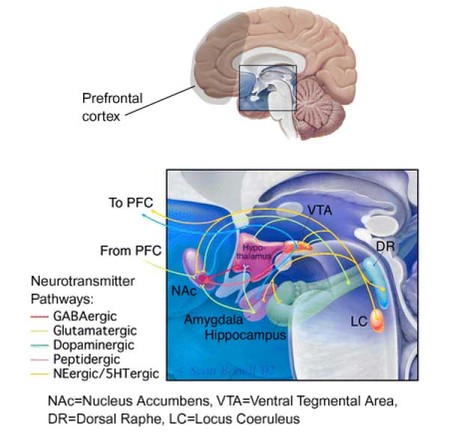
Adapted from Nestler, E. (2002) Neurobiology of depression. Neuron, 34, 13-25.
This research is informative because it suggests regions of the brain that are associated with impaired decision-making. The findings will be enhanced by new technology and research that takes into account the connectionist context of the brain. By using fMRI (functional Magnetic Resonance Imaging) to show the anatomical correlates of behavior in the brains of GT-playing subjects, we can effectively compare deficit states in substance dependent individuals and pathological gamblers with normal neural functioning in healthy controls. In finding anatomical markers of these deficits, we can broaden our understanding of the underlying biological basis for addictive diseases.
The use of fMRI-compatible laboratory measures in finding the correlates of addicted behaviors, including decision making impairments, can serve to discover at-risk populations, diagnose likelihood of relapse, and implicate possible pharmacologic mechanisms of complex addictions.
Comments on this article can be addressed to Fred Sheahan at wager@hms.harvard.edu.
References
Bechara, A. (2003). Risky business: emotion, decision-making, and addiction. Journal of Gambling Studies, 19(1), 23-51.
Childress, A. R., Mozley, P. D., McElgin, W., Fitzgerald, J., Reivich, M., & O’Brien, C. P. (1999). Limbic activation during cue induced cocaine craving. American Journal of Psychiatry, 156, 11-18.
Fuster, J.M. (1997). The Prefrontal Cortex – Anatomy, Physiology, and Neuropsychology of the Frontal Lobe, Third Edition (Philadelphia: Lippincott-Raven).
Fuster, J. M. (2001). The Prefrontal Cortex – An Update: Time Is of the Essence. Neuron, 30, 319-333.
Goldman-Rakic, P.S., Scalaidhe, S.P.O., and Chafee, M.V. (2000). Domain specificity in cognitive systems. In The New Cognitive Neurosciences, M.S. Gazzaniga, ed. (Cambridge, MA.: MIT Press), pp.733-742.
Harlow, J. M. (1868). Recovery from the passage of an iron bar through the head. Publications of the Massachusetts Medical Society, 2, 327-347.
Potenza, M., N., Steinberg, M.,A., Skudlarsky, P.,
Fulbright, R.,K., Lacadie, C.,M., Wilbur, C.,K., Rounsaville, B.,J., Gore, J.,C., & Wexler, B.,E. (2003). Gambling urges in pathological gambling: A functional magnetic resonance imaging study. Archive of General Psychiatry, 60(8), 828-836.
The WAGER is a public education project of the Division on Addictions at Harvard Medical School. It is funded, in part, by the National Center for Responsible Gaming, the Massachusetts Department of Public Health, the Substance Abuse and Mental Health Services Administration, and the Center for Substance Abuse Treatment.




Evelyn Wangari June 5, 2017
Informative topic.
Evelyn Wangari June 7, 2017
This is well said