Last year, the WAGER 3(23) discussed the use of naltrexone as a possible pharmacological treatment for pathological gambling. Naltrexone, also known by its trade name, ReVia, is an opioid antagonist produced by Dupont Pharma. Like other opioid antagonists, naltrexone binds to opioid receptors in the brain. This action protects these receptors from the binding action of opiates (e.g., heroin and morphine). Used properly, naltrexone effectively denies the patient the euphoric effects that normally accompany the administration of opioids. Naltrexone is also an adjunct therapy for those suffering with alcoholism; it decreases alcohol craving and, therefore, consumption. Consequently, it appears helpful in preventing relapses. However, the pharmacodynamics of the drug for this indication are not fully understood.1
One preliminary study reports the efficacy of naltrexone for treating impulse control disorders, the meta-category in which pathological gambling is classified by DSM-IV.2 Naltrexone has disadvantages, too. A primary side effect is nausea, which caused 15% of study subjects to drop out. Perhaps more serious is the potential hepatotoxicity of naltrexone, which contraindicates use with patients who have liver problems. This property is particularly unfortunate given the high potential for liver dysfunction among abusers of alcohol. For a pharmacotherapy to be a viable treatment option, such contraindications and side effects must be eliminated or greatly reduced.
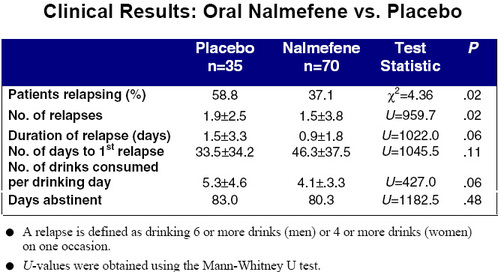
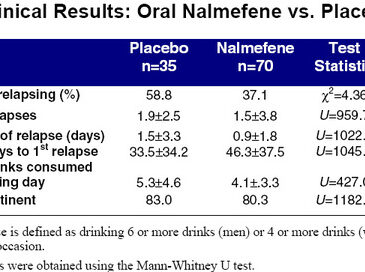
Enter nalmefene, dispensed under the trade name Revex. Recognizing the problems associated with naltrexone, Salvato, Williams, Ritvo, and Cutler (1999) conducted a double-blind, placebo-controlled study of this newer opioid antagonist.3 Chemically similar to naltrexone, nalmefene reportedly lacks the hepatotoxicity of its analogue while having superior pharmacodynamic properties. A total of 105 patients between the ages of 18 and 65 were randomized after being extensively screened. Thirty-five subjects were given placebo, and 70 were given daily doses of either 20 or 80mg. Although nalmefene is only marketed in an injectable form, the authors obtained a supply of tablets directly from the manufacturer for use in this study. The results of this trial are presented in the two accompanying tables. 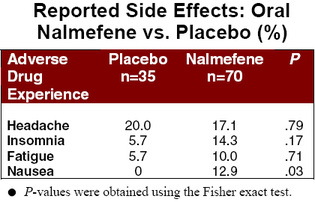
The patients of nalmefene experienced significantly fewer relapses than the placebo group. A similar trend was observed for total number of relapses. And, although inter-group differences in duration of relapse and number of drinks consumed failed hypothesis testing, testing, these results approached statistical significance. No statistically significant difference was found for the number of days abstinent. With the noteable exception of nausea, subjects on nalmefene were no more likely than the placebo group to experience side effects.
The authors correctly note that subjects enrolling in clinical trials such as this one may be more motivated to succeed than persons with addictions in the general population. This selection bias has the potential to inflate efficacy indicators. However, the results are promising. And, given the apparent usefulness of opioid antagonists such as naltrexone for impulse disorders, it is possible that nalmefene will prove useful for pathological gambling as well. However, neither naltrexone or nalmefene are "magic bullet" cures. Even when these drugs are efficacious and well-tolerated, they should be used only in conjuction with behavioral treatments and other non-pharmacological therapies.
Source: 1Medical economics company. (1999). Physicians’ desk reference. Montvale, NJ: Author. 2Kim, S.W. (1998). Opioid antagonists in the treatment of impulse-control disorders. Journal of Clinical Psychiatry, 59(4), 159-164. 3Mason, B.J., Salvato, F.R., Williams, L.D., Ritvo, E.C. & Cutler, R.B. (1999). A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Archives of General Psychiatry, 56, 719-724.
The WAGER is funded, in part, by the National Center for Responsible Gaming, the Massachusetts Department of Public Health, the Andrews Foundation, the Addiction Technology Transfer Center of New England, the Substance Abuse and Mental Health Administration Services, and the Center for Substance Abuse Treatment.