ASHES, Vol. 7(2) – Slight prick, all better? Stage II trial results of a nicotine vaccine
Nicotine vaccines represent a potentially important treatment option for quitting and preventing tobacco smoking. Nicotine vaccines work by inducing the immune system to create antibodies (Ab) that bind to nicotine in the bloodstream and prevent it from reaching the brain. By keeping nicotine from the reward pathways in the brain, nicotine Ab eliminate the pleasurable (i.e., reinforcing) aspect of smoking (Hatsukami, et al., 2011). Today’s BASIS examines the results from the recently completed Stage II trials of NicVAX, the vaccine furthest along in development (Hatsukami, et al., 2011).
Methods
- This study employed a double-blind, placebo-controlled design.
- 301 participants responded to recruiting advertisements across nine US sites.
- A total of 194 participants completed the study. All participants reported smoking more than 15 cigarettes per day and expressed motivation to quit smoking.
- Researchers randomly assigned participants to experimental (i.e., one of five possible doses of NicVAX) and control (i.e., placebo) conditions.
- Using an electronic diary, participants recorded their cigarette use and researchers collected blood and urine to objectively measure cigarette consumption.
- The study utilized blood antibody concentration as the independent variable.
- Researchers divided participants into three groups: high Ab (top 30% of participants; n = 38), low Ab (remaining 70% of those who received vaccine; n = 89) and placebo (n = 67).
Results
- The high Ab group was roughly twice as likely to achieve eight weeks of complete abstinence (24.6%) compared to the placebo group (12.0%; p = .024, odds ratio (OR) = 2.69, 95% CI [1.14-6.37]). However, low Ab participants (9.3%) did not differ statistically from the placebo group (p = .46). The high Ab group also was about two and a half times as likely to remain abstinent 26 weeks after the final infusion (19.7%) compared to the placebo group (10.0%; p = .44, OR = 2.46, CI [1.53-7.13]). Again, the low Ab group (7.1%) did not differ from the placebo group (10.0%, p = .43). Researchers defined abstinence as self-reported abstinence confirmed by blood and urine testing. Figure 1 shows the rates of 8-week abstinence for the three analysis groups.
- Participants in the high Ab group who did not completely abstain from smoking smoked on average 4.6 fewer cigarettes per person per day compared to placebo participants. The authors do not report the difference compared to the low Ab group.
- Participants did not report any differences in withdrawal symptoms across groups.
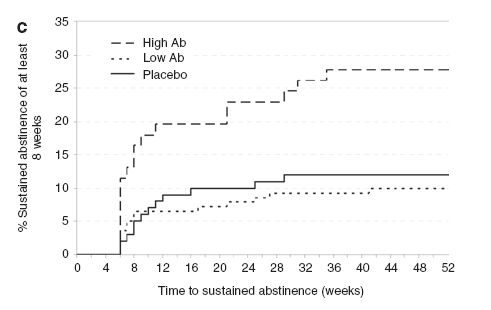
Figure. Rates of eight-week continuous abstinence for the three analysis groups. Adapted from Hatsukami et al. (2011). Click image to enlarge.
Limitations
- All participants expressed motivation to change, and the vast majority had previously attempted to quit smoking. These results might not generalize to smokers who are not motivated to quit.
- The investigators used vaccine dosages that only yielded high Ab for 30% of the sample. The study does not provide a recommended dosage regimen to reliably reach therapeutically relevant Ab levels.
Discussion
Although not a miracle cure, NicVAX shows promise as a treatment option. Six month abstinence rates for those in the high Ab group are more than twice as high as those reported for nicotine replacement therapy and four times higher than those who attempt to quit ‘cold turkey’. Further research and development is needed to perfect precise dosage regimens, but NicVAX might be a very useful tool in the arsenal of treatment options against nicotine addiction. If stage III trials run smoothly, NicVAX may be FDA approved by the end of 2011 and available as a treatment by 2012 (Drugwatch.com, 2009).
-Daniel Tao
References
Drugwatch.com. (2009). Nicotine Vaccine NicVAX is first to reach late-stage trials. Retrieved Feb. 24, 2011, from http://www.drugwatch.com/news/2009/11/12/nicotine-vaccine-nicvax-first-reach-late-stage-trials/
Hatsukami, D. K., Jorenby, D. E., Gonzales, D., Rigotti, N. A., Glover, E. D., Oncken, C. A., et al. (2011). Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clinical Pharmacology & Therapeutics, 89(3), 392-399.
What do you think? Please use the comment link below to provide feedback on this article.