ASHES, Vol. 6(4) – Even better than the real thing? Electronic cigarettes vs. real cigarettes
Despite a lack of research, electronic cigarette (e-cigarette) companies market their products as “safe,” without harmful carcinogens found in cigarettes, and helpful in the cessation process (World Health Organization, 2008, 2009). E-cigarettes are a refillable nicotine cartridge with a heater that vaporizes a nicotine solution (i.e., nicotine, propylene glycol and other chemicals), which then gets absorbed into the lungs when the smoker takes a “puff” (Laugesen, 2009; World Health Organization, 2008). This week the ASHES reviews a study that compares participants’ nicotine and craving levels from smoking their usual cigarette brand to levels produced by two e-cigarettes (Eissenberg, 2010).
Methods
- The researcher used a convenience sample to identify 16 eligible participants.
- Eligibility criteria included:(1) healthy adults aged 18-55 who smoked > 15 cigarettes/day for at least 1 year; (2) no knowledge of e-cigarettes; (3) not trying to quit smoking; and (4) agreed not to use tobacco/nicotine 12 hours prior to each session (confirmed by < 10 ppm expired air CO).
- Participants went to one session for each of the four products (separated by 48 hours): preferred cigarette brand, sham cigarette (puffing on preferred unlit cigarette), and two e-cigarettes (Brand One had a 18mg nicotine cartridge and Brand Two a 16mg cartridge).
- Dependent measures included:
- Blood plasma nicotine levels; and
- Craving – (1) Tiffany-Drobes Questionnaire of Smoking Urges Brief (QSU Brief); and (2) a 35-item computerized visual analog scale (VAS) consisting of the Hughes-Hatsukami Smoking Withdrawal Scale (Hughes & Hatsukami, 1986).
- The researcher obtained these measures 5 minutes prior to the first puff and 5, 15, 30 and 45 minutes after the first puff (bout 1). After 60 minutes, the product was administered again and the process was repeated (bout 2).
Results
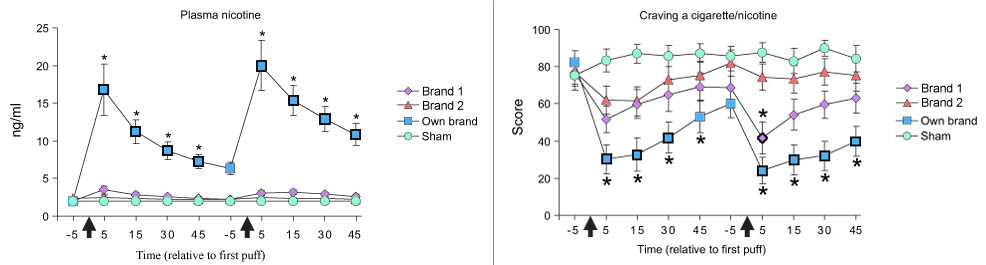
- The Figure shows that the lit preferred brand cigarette significantly increased plasma nicotine and decreased cigarette craving at most recorded timepoints (p < 0.05) during bouts 1 and 2.
- The two e-cigarettes failed to significantly increase plasma nicotine levels and decrease cigarette craving at all recorded timepoints during bouts 1 and 2, except Brand One significantly decreased cigarette craving at the 5 minute timepoint during bout 2 (p < 0.05).
- The sham cigarette failed to significantly increase plasma nicotine levels and decrease cigarette craving at all recorded timepoints.
Figure. Mean Plasma Nicotine Level and Craving a Cigarette/Nicotine Score for each Product (from Eissenberg, 2010). Click image to enlarge.
Note: Arrows indicate the time the product was administered (10 “normal” puffs with 30 second intervals between puffs). Bold symbols indicate a significant difference from the first timepoint following product administration. Asterisks above or below the symbol indicate a significant difference from sham smoking at the given timepoint (p < 0.05).
Limitations
- The sample size is small and from a convenience sampling strategy, making it difficult to generalize to all smokers.
- Vapor content can differ based on the e-cigarette design.
- Nicotine delivery can vary among participants based on their chronic use and intensity of inhalation.
Discussion
Both brands of e-cigarette reduced nicotine use by delivering less nicotine than the products’ claimed, but neither significantly reduced craving. Therefore, smokers might be more likely to revert to smoking their preferred brand of cigarette to satisfy their craving and expose themselves to the harmful chemicals associated with cigarette smoking. If manufacturers want to market e-cigarettes as a harm reduction tool, then these results suggest that e-cigarette companies might want to do additional testing and product revisions to ensure that they effectively reduce cravings.
-Tasha Chandler
References
Cobb, C. O., Weaver, M. F., & Eissenberg, T. (2009 Online First). Evaluating the acute effects of oral, non-combustible potential reduced exposure products marketed to smokers. Tobacco Control, doi: 10.1136/tc.2008.028993.
Eissenberg, T. (2010). Electronic nicotine delivery devices: Ineffective nicotine delivery and craving suppression after acute administration. Tobacco Control, 19(1), 87-88.
Hughes, J. R., & Hatsukami, D. (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43(3), 289-294.
Laugesen, M. (2009). Ruyan e-cigarette bench-top tests. Poster presented to the joint conference of the Society for Research on Nicotine and Tobacco and Society for Research on Nicotine and Tobacco-Europe; April 30, 2009.
World Health Organization. (2008). Marketers of electronic cigarettes should halt unproved therapy claims. Retrieved April 14, 2010, from http://wno.int/mediacentre/news/releases/2008/pr34/en/index.html
World Health Organization. (2009). The scientific basis of tobacco product regulation: Report of a WHO Study Group. Geneva, Switzerland: World Health Organization.
What do you think? Please use the comment link below to provide feedback on this article.