The WAGER, 8(36) – Biology, Addiction, and Gambling: What We Can Learn from the Rat Race
As noted in previous issues of The WAGER (8(30) – 8(35)), most drugs associated with addiction affect the release and regulation of dopamine in the brain’s “reward system” – a set of neural pathways involved in the experience of pleasure. Past research has focused on chemical objects of addiction, but evidence is emerging to imply that behavioral objects of addiction (e.g., shopping, gambling, etc.) might hold similar sway over the reward system (Betz, Mihalic, Pinto, & Raffa, 2000; Blum et al., 2000; Breiter, Aharon, Kahneman, Dale, & Shizgal, 2001; Potenza, 2001). This suggests that seemingly disparate patterns of addiction, such as cocaine addiction and gambling addiction, might actually stem from a common root. This week’s WAGER reviews two research papers by Werme and his colleagues (Werme, Lindholm, Thoren, Franck, & Brene, 2002; Werme, Thoren, Olson, & Brene, 2000) that demonstrate the non-specificity of addiction by focusing on the similar activation of the reward system by cocaine, chronic running, and alcohol.
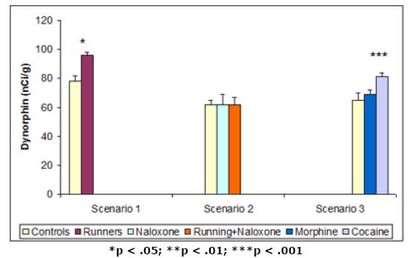
The first paper by Werme et al. (2000) tested the effects of cocaine, morphine, and chronic running on levels of a kind of mRNA known to increase dopamine receptors within the brain’s reward system1. They hypothesized that if chronic running acted on the reward system in the same way as cocaine, similar adaptations within this system (i.e., increases in the expression of the mRNA) ought to be evident. Using a strain of rats known for their affinity for running wheels and drugs (i.e., Lewis rats), the researchers ran three different scenarios. In the first, they provided 8 rats with access to running wheels and retained 8 with no access (i.e., controls). In the second, they injected saline solution into 8 rats with no access to running wheels, and injected naloxone (i.e., a chemical that blocks the drug-induced process leading to the increase of mRNA) into 6 rats with access and 6 rats with no access. In the third, in which no rats had access to running wheels, they injected 7 rats with saline, 7 rats with morphine, and 7 rats with cocaine. In all cases, they measured levels of mRNA in areas of the brain’s reward pathway after the experiment. The results from these three scenarios are shown in Figure 1.
Figure 1. Dynorphin mRNA levels after (1) running, (2) running + naloxone injection, and (3) cocaine injection (Adapted from Werme et al., 2000.)
In the first scenario, the rats given access to running wheels had higher levels of mRNA in their reward pathways than those without access. In the second scenario, injection of naloxone erased this effect. In the third scenario, injection of cocaine showed the same positive effect on mRNA levels as chronic running. All of these results indicate that chronic running not only looks behaviorally similar to a drug addiction, but also acts on the brain in a neurochemically similar fashion. Chronic running increases levels of dopamine, resulting in the pleasure associated with addiction, and causes the brain to adapt to the increased level of dopamine by producing more mRNA and consequently creating more receptors. This eventually can result in tolerance and withdrawal symptoms.
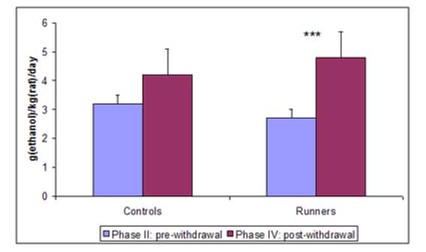
In their second paper, Werme et al. (2002) tested whether running could increase response to another drug. Since most drugs associated with addiction act on the same system in the brain, the action of one object of addiction might sensitize the system to the effects of another – a process called potentiation. Such sensitization might be evidenced by increases in addiction to and preference for the objects. Again, Werme et al. (2002) used Lewis rats to test their hypothesis. First (phase I), they gave all rats access to running wheels for two weeks. Second (phase II), they gave them access to alcohol but no running wheels for five weeks and measured their intake and preference (for alcohol vs. water). Third (phase III), they split the rats into two groups – one with access to running wheels and one without – and withdrew all alcohol for a week. Finally (phase IV), they gave both groups free access to alcohol for a week and measured their intake and preference. The results are shown in Figure 2.
Figure 2. Alcohol intake (left) and preference (right) before and after withdrawal in runners and controls. (Adapted from Werme et al., 2002.)
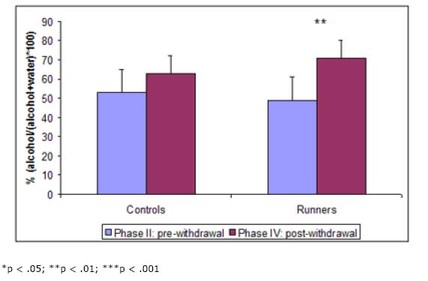
In this experiment, chronic runners showed an increased preference for alcohol. This result, in conjunction with the other presented findings, provides a compelling picture of the similar neural pathways and mechanisms of drug and behavioral addictions. It also supports clinical reports of addicts’ tendency to “addiction hop” – substitute one addiction for another during recovery.
Because of the nature of animal research about human psychology, several limitations apply: (1) these findings are only applicable to the extent that the rat brain resembles that of a human; (2) the study uses rats genetically prone to addiction; and (3) the results cannot be directly related to gambling so long as rats prefer a wheel and some sawdust to a hand of cards. That said, this research contributes evidence about the neurobiological underpinnings of addiction and the surprising neural similarity of chronic behaviors to drug addiction. Such similarity implies that addiction concerns not so much specific drugs or objects, but opportunity, experience, and process.
Comments on this article can be addressed to Sarah Nelson at wager@hms.harvard.edu.
Notes
1 To understand this research, a basic knowledge of addictive drugs’ effects on the brain’s reward system is necessary. Most addictive drugs affect the release and regulation of dopamine in this system. The “high” experienced is a result of dopamine being released. Extended use actually affects the genetic coding for dopamine receptors in this system, leading to symptoms of tolerance and withdrawal. The brain essentially adapts to the increased level of dopamine in this system (e.g., by increasing the number of dopamine receptors) so that the same amount of drug use that produced the original “high” has no effect, and ceasing drug use has a negative effect.
References
Betz, C., Mihalic, D., Pinto, M. E., & Raffa, R. B. (2000). Could a common biochemical mechanism underlie addictions? Journal of Clinical Pharmacy & Therapeutics, 25(1), 11-20.
Blum, K., Braverman, E. R., Holder, J. M., Lubar, J. F., Monastra, V. J., Miller, D., et al. (2000). Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs, 32(S), 1-112.
Breiter, H. C., Aharon, I., Kahneman, D., Dale, A., & Shizgal, P. (2001). Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron, 30(2), 619-639.
Potenza, M. N. (2001). The neurobiology of pathological gambling. Seminars in Clinical Neuropsychiatry, 6(3), 217-226.
Werme, M., Lindholm, S., Thoren, P., Franck, J., & Brene, S. (2002). Running increases ethanol preference. Behavioural Brain Research, 133, 301-308.
Werme, M., Thoren, P., Olson, L., & Brene, S. (2000). Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. European Journal of Neuroscience, 12, 2967-2974.
The WAGER is a public education project of the Division on Addictions at Harvard
Medical School. It is funded, in part, by the National Center for Responsible
Gaming, the Massachusetts Department of Public Health, the Substance Abuse and
Mental Health Services Administration, and the Center for Substance Abuse
Treatment.